جامعة الكفيل
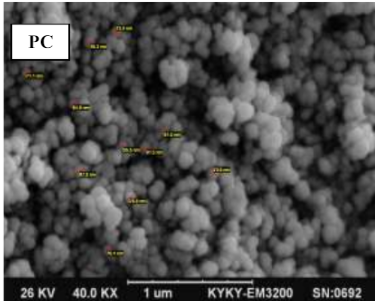
In this study, the carbon nanospheres were synthesized by burning diesel and then treated with hydrogen peroxide (H2O2). The results showed that H2O2 - carbon nanospheres has a higher surface area than carbon nanospheres and therefore it was chosen as an adsorbent surface to remove the Rose Bengal dye from aqueous solutions. The prepared carbon nanospheres were examined by FTIR, XRD, SEM, AFM, TGA, Raman spectroscopy, BET and EDX. The FTIR study reveals the presence of hydroxyl and carboxyl stretching vibration and weak peaks belong to CH3, CH2 and C=C. Results obtained by Raman and XRD analysis are in good agreement thereby indicating the amorphous structure of the carbon nanospheres. Also, SEM images confirm the presence of soot materials as spherical and semispherical nanoparticles with diameter in the range (31-78 nm). Surface roughness calculated from AFM data provided evidence that spiky appearance on both carbon surfaces. TGA data indicate that both carbon samples are thermally unstable. BET and BJH results indicate that the treated sample possesses the highest surface area and mean pore diameter. EDX analysis indicated the presence of pure carbon nanosphere (treated sample) without any contamination. Also, the adsorptive removal of Rose Bengal on synthesized carbon nanospheres was studied. The isotherm adsorption results were found to be described fitted by the Freundlich rather than the Langmuir and Temkin models. Furthermore, the kinetics of dye adsorption were applied better by the pseudo-second-order model.

In this study, the carbon nanospheres were synthesized by burning diesel and then treated with hydrogen peroxide (H2O2). The results showed that H2O2 - carbon nanospheres has a higher surface area than carbon nanospheres and therefore it was chosen as an adsorbent surface to remove the Rose Bengal dye from aqueous solutions. The prepared carbon nanospheres were examined by FTIR, XRD, SEM, AFM, TGA, Raman spectroscopy, BET and EDX. The FTIR study reveals the presence of hydroxyl and carboxyl stretching vibration and weak peaks belong to CH3, CH2 and C=C. Results obtained by Raman and XRD analysis are in good agreement thereby indicating the amorphous structure of the carbon nanospheres. Also, SEM images confirm the presence of soot materials as spherical and semispherical nanoparticles with diameter in the range (31-78 nm). Surface roughness calculated from AFM data provided evidence that spiky appearance on both carbon surfaces. TGA data indicate that both carbon samples are thermally unstable. BET and BJH results indicate that the treated sample possesses the highest surface area and mean pore diameter. EDX analysis indicated the presence of pure carbon nanosphere (treated sample) without any contamination. Also, the adsorptive removal of Rose Bengal on synthesized carbon nanospheres was studied. The isotherm adsorption results were found to be described fitted by the Freundlich rather than the Langmuir and Temkin models. Furthermore, the kinetics of dye adsorption were applied better by the pseudo-second-order model.